● K2M company and 3D Systems team created a new type of interbody fusion cage for spinal diseases, exploring patient-oriented implants
- Quick understanding --
Challenge:
Provide new treatments for spinal diseases
Solution:
K2M's rich experience in spinal diseases is combined with 3D Systems' metal 3D printing
Results:
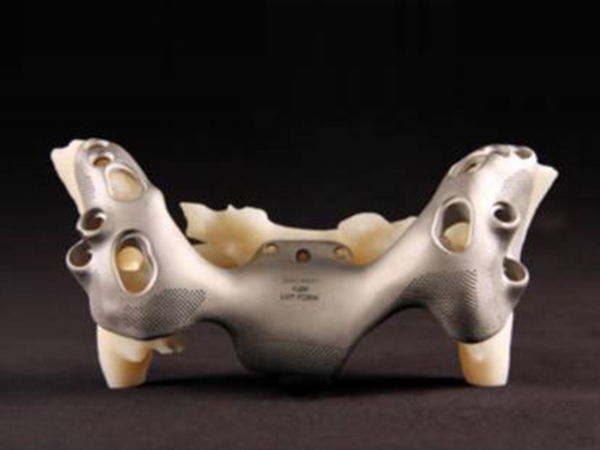
● K2M's fast-growing product CASCADIA Interbody Systems has porous characteristics and a certain roughness on the surface, which helps bone growth
● Use virtual surgery planning VSP to make 3D printed "licensed use" implants for patients who currently do not have better treatment methods
● Extend 3D printing to other areas, such as new patient-specific spinal implants
"1"
Let’s first understand how K2M and 3D Systems sparked.
If both parties have their own strengths and cooperate closely, the final result will be 1+1 greater than 2. The cooperation between K2M and 3D Systems fully proves this, and the common values of the two companies have strengthened the innovation of technological processes and results.

K2M is a company dedicated to complex spinal products and minimally invasive spinal surgery solutions. Doctors around the world use K2M's products to treat patients with complicated spinal diseases.
K2M hopes to develop solutions to spinal problems in depth. They want to find advanced medical device manufacturers that can match their expertise in spinal juice treatment.
As an industry leader, 3D Systems not only has more than 30 years of rich printing experience, but also often provides VR surgery simulators and 3D printed anatomical structures for surgeons, medical workers, medical device manufacturers and medical teaching staff familiar with precision medical solutions. Models, virtual surgical planning VSP, patient-specific 3D printed surgical guides, instruments and implants.
"2"
It's a hit
Jim Han, the head of K2M's complex spine department, said: "The cooperative relationship between K2M and 3D Systems is thriving based on the technological advantages and common cultural values of both parties."
"In the field of spine, we are experts; in the field of 3D printing, 3D Systems is an expert. We have a consensus on how to operate, investment in innovation, overall goals, and cure more patients."
K2M was originally a company that provided new technologies and medical devices for the treatment of spinal deformities. After the introduction of 3D printing, K2M's market share in spinal deformity treatment devices (the treatment of spinal degeneration through a more minimally invasive method) has grown rapidly.
"We need 3D printing to help develop spinal degeneration and minimally invasive treatments. 3D Systems gives us this ability. Our cooperation with 3D Systems allows us to maintain a leading position in the manufacturing of 3D printed spinal therapy devices, which allows us We can explore more deeply in the field of spinal therapy. In the past few years, our growth has been very rapid."
K2M's 3D printed implants are made through 3D Systems' Direct Metal Printing (DMP). K2M's implants have been used to explore new areas, and the application of metal printing technology to precision medicine has been previously verified.
Van "Kleine Borugel said:" More than ten years of experience and 500,000 manufactured devices have made us realize that metal printing is very suitable for making spinal fusion cages with porous structures and organic shapes. "
"3"
3D printed implants promote bone growth
3D Systems provides design consulting, 3D printing production and post-processing for the FDA-certified CASCADIA series of implants. The fast-growing CASCADIA product line under K2M also won the Orthopedic Week Spine Technology Award.
CASCADIA products use 3D Systems' ProX DMP 320 printer and K2M, an advanced method called Lamellar 3D Titanium Technology (Lamellar 3D Titanium Technology) to create structures that cannot be made by traditional methods.

3D Systems’ ProX DMP 320 printer and K2M’s Lamellar 3D Titanium Technology (Lamellar 3D Titanium Technology) made implant CASCADIA? Interbody Systems, which has a certain porosity and surface roughness, which is conducive to bone growth.
Using titanium powder, the implant is made with a high-energy laser beam, with a certain pore and surface roughness for bone growth. The manufactured implant has the strength of titanium, but it does not hinder the X-ray irradiation, so as to give the doctor a better observation perspective and understand how to interact with the patient's body structure.
Direct metal printing (DMP) is very suitable for making metal parts with porous structures and organic shapes, such as K2M's spinal fusion cage

Lamellar 3D Titanium Technology in the K2M product portfolio includes CADCADIA TL, AN, Lateral, AN Lordotic Oblique, and Cervical Interbody Systems products. For the production process of these products, 3D Systems not only performs 3D printing, but also performs post-processing, such as heat treatment, surface finishing, cleaning and laser marking. In short, what customers need, we meet customer needs.

3D Systems' ProX 320 printer

Other metal parts made by ProX 320 printer
"4"
Practical use of 3D printed implants
Tom Morrison, a physician at the North Star Spine and Neurosurgery Center in the United States, has used CASCADIA Interbody Systems to treat patients for more than a year. Morrison said: “I am very impressed with Lamellar 3D Titanium Technology. The porosity and surface roughness are biologically feasible. In the long-term follow-up of the patient, the patient is also The response is good. In the future, I hope to continue to use the implant CASCADIA Interbody Systems, and I also look forward to seeing K2M's layered 3D titanium technology be used in more bone growth fields.
In a recent case of cooperation between K2M and 3D Systems, through the collaboration of 3D Systems' virtual planning surgical system VSP and K2M, a "licensed use" implant was created for patients that cannot be treated by current medical treatment.
Sean Reynolds, a project manager of K2M, talked about the company's recent research and development results with the University of Colorado neurosurgery doctor Michael Fein who specializes in spinal diseases.
Dr. Fein has a patient situation that is suitable for the use of 3D printed implants. This patient had had implant surgery before, but the postoperative condition was not good and his movement was restricted by pain. At the same time, there are no better alternatives on the market to relieve his symptoms. Dr. Fein believes that the hardware implanted in the past surgery will be removed, and 3D printed implants will be used as an alternative treatment.

K2M and 3D Systems have worked together to produce a 3D printed "licensed use" implant, which is a model for the future application of metal 3D printing in the medical field
Dr. Fein said: “The implants that the patient had put on his body put a lot of pressure on his body, and the patient’s recovery is not optimistic. I think 3D printed implants can give customers better treatment results.”
Through spinal scans and online meetings with K2M and Dr. Fein, 3D Systems conducted a virtual surgical planning for this operation. Their communication mainly revolves around the design of the implant and the positioning and type of the implant.
Dr. Fein said that the operation was a success and the cooperation between K2M and 3D Systems was very smooth.
"The positive response of these two companies impressed me. They pay attention to the timeliness of the completion of things. K2M knows how to innovate to support doctors in completing complex operations, and 3D Systems has experienced many similar operations in the past. I'm already very skilled."
"5"
Imagine the future of medical care with 3D Systems
For Sean Reynolds and K2M, from the preparatory stage to the successful operation, this is an exciting prelude, and it is also a potential business area with great potential: providing surgical planning and use for complex spinal degeneration diseases Custom implants.
Although there are fewer cases of "licensed use", the above case will be a model for future custom implants. In the future, it will no longer be new to make FAD-approved implants based on the individual needs of patients.
Reynolds believes: "Based on our 3D printing experience, we believe that the application of 3D printed implants will be more common in the future, not only for complex cases, but also for spinal degenerative diseases. We are very optimistic about 3D implants. I believe that 3D Systems’ extension in the medical field will be broader."
![]() Tel:+86 135 7086 9158
Tel:+86 135 7086 9158![]() Tel:+86 135 7086 9158
Tel:+86 135 7086 9158